Successful Synthesis of Rare Isotopic Atropisomers with High Rotational Stability
2022/10/26
- Research
A research team from Japan synthesized isotopic atropisomers based on ortho-CH3/CD3 discrimination, which demonstrate high rotational stability and stereochemical purity
The detection and synthesis of isotopic atropisomers is difficult due to their low rotational stability and very slight difference in the chemical property between their H and D atoms. Now, for the first time, researchers from Japan have succeeded in synthesizing diastereomeric 3-(2-trideuteriomethyl-4,6-dimethylphenyl)-2-(1-phenylpropan-2-yl)quinazolin-4-ones, i.e., isotopic atropisomers, based on ortho-CH3/CD3 discrimination. These quinazolinone derivates demonstrated high rotational stability, high optical purity, and slight optical rotation.
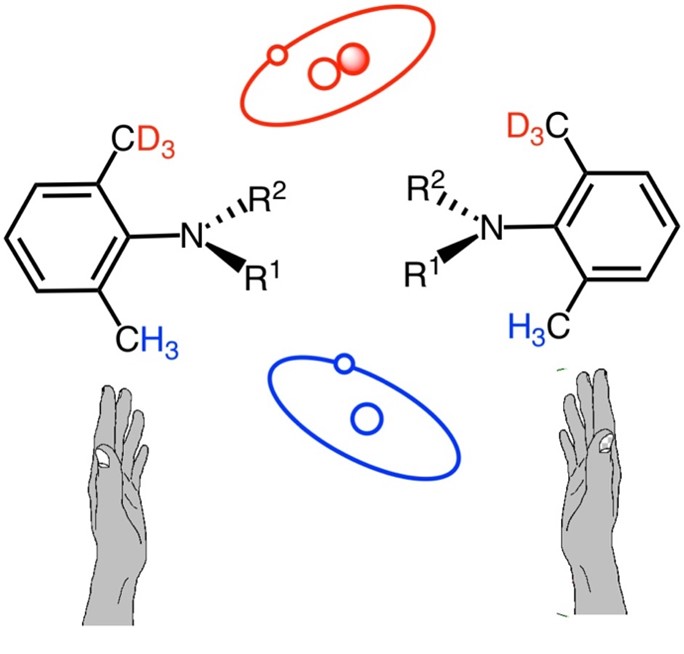
Image title:Isotopic atropisomers based on ortho-CH3/CD3 discrimination
Image caption:The image depicts isotopic atropisomers based onCH3/CD3 discrimination. The left and right hands indicate the inherent chirality in the system. A new study conducted by researchers from Shibaura Institute of Technology is one of the first to lead to the successful preparation of isotopic atropisomers with high rotational stability and slight optical rotation, based on ortho-CH3/CD3 discrimination.
Image credit:Dr. Osamu Kitagawa from Shibaura Institute of Technology
Image license:Original content
Image caption:The image depicts isotopic atropisomers based onCH3/CD3 discrimination. The left and right hands indicate the inherent chirality in the system. A new study conducted by researchers from Shibaura Institute of Technology is one of the first to lead to the successful preparation of isotopic atropisomers with high rotational stability and slight optical rotation, based on ortho-CH3/CD3 discrimination.
Image credit:Dr. Osamu Kitagawa from Shibaura Institute of Technology
Image license:Original content
In contrast to well-known centrally chiral deuterated compounds, isotopic atropisomers, i.e., compounds formed as a result of sterically hindered rotation around a single bond, are very rare. At present, isotopic atropisomers with high rotational stability and stereochemical purity have not been reported.
The detection of isotopic chiral compounds is generally difficult owing to the minute differences between the chemical and physical properties of their H and the D atoms. In the case of atropisomers, this difficulty is enhanced further, due to their low rotational stability and the spatial distance between their H and D atoms.
Recently, a team of researchers from Japan, including Professor Osamu Kitagawa of Shibaura Institute of Technology and his students Shota Miwa and Ryunosuke Senda, conducted a study to synthesize isotopic atropisomers with high rotational stability and optical purity. Their paper, published in The Journal of Organic Chemistry on October 10, 2022 was chosen as one of the four featured articles of 2022, from over a thousand publications in this journal.
“I have always been interested in preparing organic molecules with unique structures. I was particularly curious about the verification of rotationally stable isotopic atropisomers,” says Dr. Kitagawa while discussing his motivation behind the study.
For the study, the team used N—C axially chiral 3-(2-bromo-4,6-dimethylphenyl)-2-ethylquinazolin-4-one—a racemic mixture, i.e., a mixture that contains equal amounts of left and right enantiomers. First, they conducted kinetic optical resolution of the racemic mixture through a chiral palladium-catalyzed hydrodebromination reaction followed by optical purification to get optically pure enantiomers. Subsequently, Suzuki-Miyaura cross-coupling of the enantiomers with CD3B(OH)2 gave the desired 3-(2-CD3-6-methylphenyl)quinazolin-4-ones possessing a slight optical rotation. However, since the prepared enantiomers could not be separated through high-performance liquid chromatography (HPLC) using a chiral column, their optical purity and rotational stability were not determined. This led the team to prepare the diastereomeric quinazolinone derivatives.
As a result, the diastereomeric 3-(2-trideuteriomethyl-4,6-dimethylphenyl)-2-(1-phenylpropan-2-yl)quinazolin-4-ones with an atropisomerism and chiral carbon were prepared.
Through proton nuclear magnetic resonance (NMR) measurements, the team detected a clear difference of ortho-methyl hydrogens between the diastereomers (the isotopic atropisomerism based on CH3/CD3 discrimination). In addition, they found that the prepared diastereomeric quinazolinones have high enantio- and diastereomeric purity and high rotational stability.
“Our work is the first to demonstrate the asymmetric synthesis of isotopic atropisomers and to verify rotationally stable isotopic atropisomerism. These results might be too preliminary to contribute to real-life applications soon. However, I sincerely hope that more scientists get inspired by our work and contribute to similar research,” concludes Dr. Kitagawa, while discussing the relevance of these findings.
Here’s hoping that we continue to witness the preparation of unique organic compounds through exceptional research!
Reference
| Title of original paper: | Asymmetric Synthesis of Isotopic Atropisomers based on ortho-CH3/CD3 Discrimination and Their Structural Properties |
| Journal | The Journal of Organic Chemistry |
| DOI: | 10.1021/acs.joc.2c02185 |
Funding Information
This work was partly supported by JSPS KAKENHI Grant umber C 20K06945.Contact
Planning and Public Relations Section
3-7-5 Toyosu, Koto-ku, Tokyo 135-8548, Japan (2F the Centennial Main Building, Toyosu Campus)
TEL:+81-(0)3-5859-7070 / FAX:+81-(0)35859-7071
E-mail:koho@ow.shibaura-it.ac.jp