New Imaging Technique for Early Detection of Blood Trauma
2021/12/16
- Research
Scientists develop a new method to analyze red blood cell deformities as a marker for shearing stress-induced cell damage
Medical devices, such as artificial organs, often cause damage to RBCs in the bloodstream owing to a high level of “shearing stress.” Conventional methods for assessing blood damage examines damaged RBCs. But is it possible to detect this damage earlier and reverse it? A global team of scientists recently developed a new image analysis method that detects RBC distortions before they are completely destroyed, opening doors to an early diagnosis of sublethal blood trauma.
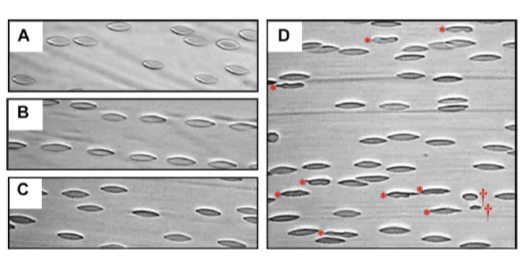
RBCs flowing under shear stresses of (A) 10 Pa, (B) 30 Pa, (C) 40 Pa and (D) 60 Pa.
Researchers from SIT, Japan propose a new method for detecting the presence of abnormal red blood cells that can enable an early diagnosis of onset of blood damage caused by cardiovascular support devices.
Image credit: Dr. Antony P. McNamee from GU, Australia.
Usage Restriction: Journal copyrighted
Conventional methods for examining blood damage involves using RBCs that are already damaged beyond repair. This raises a pertinent question: is it possible to detect the onset of damage at an earlier stage and reverse it? It is evident that a real-time monitoring of shear-stressed RBCs could help us better understand the process and aid in the development of markers to detect the onset of blood damage at a sublethal stage.
In their new study published in Scientific Reports, a team of researchers from Japan and Australia, led by Associate Prof. Nobuo Watanabe from Shibaura Institute of Technology, Japan and Associate Prof. Michael J. Simmonds from Griffith University (GU), Australia, devised a new approach for detecting changes that RBCs undergo in shear flow. Unlike previous studies that inspected RBCs in a static condition, this study looked at the “membrane symmetry” of the stressed RBC during “supraphysiological shear” to see how much it differed from the normal one. Dr. Watanabe explains, “Conventional image analysis methods are ill-equipped to detect subtle shape changes associated with morphological alterations observed in supraphysiological flow. With our technique, it becomes possible to assess and detect these shape changes and damage.”
To achieve this feat, the team used a custom-built shear chamber mounted on a microscope equipped with a high-speed capture camera to visualise the onset of RBC destruction. They collected blood samples from two healthy male volunteers between the ages of 20 – 30 years, and subjected them to a range of shear loads ranging from 10 – 60 pascals (Pa) for 5 minutes. They next examined the RBC count and detected abnormalities on the video footage, optimizing the imaging system to improve asymmetry detection.
They noticed something remarkable – after a prolonged exposure to a stress load of 60 Pa, the RBCs became unstable, changed shape, and even disintegrated into smaller fragments. “While the release of haemoglobin represents end-point cell destruction or ‘haemolytic stage’, our data suggest that bulk fragmentation, a lethal transition for RBC, can still be observed in the ‘subhaemolytic’ 60 Pa condition,” says Dr. Watanabe, enthralled by the findings.
The team then hypothesised that irregular shear stress, rather than regular shear stress, would result in cell stiffening at low-shear exposures before its appearance was altered. To confirm this, they applied an additional 1 Pa shear after the 60 Pa exposure, observing RBC aggregates with bloated vesicle-like structures swirling in the flow. Interestingly, the “elongation index” (EI), assessing the cell’s deviation from its normal axes, showed only minimal changes after exposure.
The team believes that this technique will allow for a more detailed examination of circulating RBC structure, accelerating the research on blood damage processes caused by cardiovascular medical equipment. “The technology can be implemented in future next-generation equipment for near-real-time diagnosis of blood injury in patients who require mechanical circulatory support,” says Dr. Watanabe. Speaking of future potential applications, he adds, “Our proposed strategy focuses on early onsetting functional markers, when blood damage may still be reversible. Integration of such functional assessment markers into the deployment of cardiovascular medical devices could help guide future clinical practise of artificial organs to enhance blood compatibilityand improve patient outcomes.”
Let’s hope his visions are not too far from being realized.
Reference
| Title of original paper: | Erythrocyte morphological symmetry analysis to detect sublethal trauma in shear flow |
| Journal | Scientific Reports |
| DOI: | 10.1038/s41598-021-02936-2 |
Funding Information
This study was funded by Japan Society for the Promotion of Science, under grants JP17K01370 and JP20K12609.Contact
Planning and Public Relations Section
3-7-5 Toyosu, Koto-ku, Tokyo 135-8548, Japan (2F the Centennial Main Building, Toyosu Campus)
TEL:+81-(0)3-5859-7070 / FAX:+81-(0)35859-7071
E-mail:koho@ow.shibaura-it.ac.jp