Atropisomeric N-Aryl Quinazoline-4-Thiones with Isotopic Differences at the Ortho Position
2021/10/07
- Research
Scientists prepared atropisomeric N-aryl quinazoline-4-thione with ortho-hydrogen/deuterium discrimination
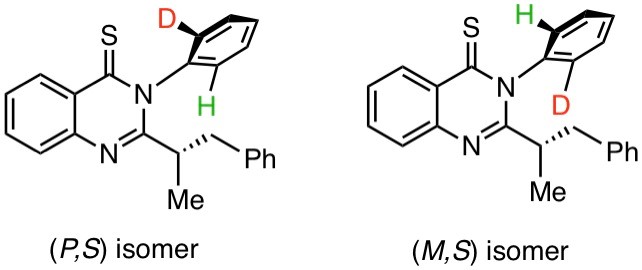
“Atropisomeres” are stereoisomers arising due to the rotational restriction about a single bond. For the rotational restriction, the substituents possessing an appropriate steric size are required around the single bond, otherwise, atropisomers are easily interconverted through the single bond rotation. Atropisomers due to Hydrogen (H) and deuterium (D) atoms have been unknown to date. Researchers from Shibaura Institute of Technology, Japan took up the challenge and synthesized 3-(2-deuteriophenyl)-2-(1-phenylpropan-2-yl)quinazoline-4-thiones.
Title: Isotopic Isomers
Caption: Scientists from SIT, Japan, found that quinazoline-4-thiones with ortho-deuterio-phenyl group possess isotopic atropisomerism based on ortho-H/D discrimination.
Photo courtesy:Osamu Kitagawa fromShibaura Institute of Technology
Usage restrictions: Copyrighted
Deuterated chiral organic compounds—molecules that are non-superimposable mirror images of each other—act as perfect probe molecules for mechanistic studies of various biochemical and synthetic reactions. Over the years, scientists have developed many reactions for the synthesis of optically active chiral deuterated compounds. However, most of them are centrally chiral compounds bearing a chiral carbon, while the synthesis of atropisomeric chiral deuterated compounds has not been reported yet because of “racemization” and difficulty of detecting the atropisomerism based on H/D discrimination.
To overcome this issue, a team of researchers from Shibaura Institute of Technology (SIT), Japan, led by Professor Osamu Kitagawa synthesized 3-(2-deuteriophenyl)-2-(1-phenylpropan-2-yl)quinazoline-4-thiones. The molecules showed unprecedented isotopic atropisomerism— a form of chirality that arises due to the difference in a bond rotation that arises from steric hindrance. The atropisomerism in the prepared quinazoline-4-one derivatives was due to rotational restriction around an N-Ar(deuterio-phenyl ) bond. As Prof. Kitagawa explains, “We were trying to understand the chemistry of N-C axially chiral compounds which is when we discovered atropisomers based on ortho-H/F discrimination. These were rare compounds where the smallest hydrogen atom and the second smallest fluorine atom were at the ortho position. This gave us an idea about creating isomers based on ortho-H/D discrimination.” The findings of their study are published in Organic Letters.
Optically active 3-(2-deuteriophenyl)-2-(1-phenylpropan-2-yl)quinazoline-4-thione was prepared via highly diastereoselective α-benzylation of a precursor [optically pure 3-(2-bromophenyl)-2-ehyl quinazolin-4-one] followed by thionation with Lawesson’s reagent and then “deuteration” using sodium boro-deutiride. This synthesis was achieved by Kazuya Saito and Shota Miwa, who are graduate students in Kitagawa’s lab.
The team (mainly Dr. Elsa Caytan at University of Rennes who is co-worker) then analyzed the samples using 1H- and 2H-NMR, which clearly indicated that the quinazoline-4-thiones existed as a 1:1 mixture of diastereomers based on a chiral carbon atom and atropisomerism due to the rotational restriction around an N-aryl bond. Density functional theory (DFT) calculations, which were performed by Dr. Yuuki Fujimoto (co-worker), also strongly supports the formation of diastereomers.
This study is the first to detect a class 2 isotopic atropisomer based on the difference in ortho-H/D. The molecules synthesized during this research could also be used for pharmaceutical purposes. “The N aryl quinazolinones and the thione analogs prepared during our study are multifunctional. Not only can they be used as probe molecules but are also of great interest from a medicinal chemistry point of view. Quinazoline-4-thiones are known to have high biological activity. They can be used to develop therapeutic agents with show antibacterial, anti-inflammatory, and anti-cancer properties,”concludes Prof. Kitagawa.
Reference
| Title of original paper: | Detection of Isotopic Atropisomerism Based on ortho-H/D
Discrimination |
| Journal | Organic Letters |
| DOI: | 10.1021/acs.orglett.1c02723 |
Funding Information
This study was partially supported by JSPS KAKENHI.Contact
Planning and Public Relations Section
3-7-5 Toyosu, Koto-ku, Tokyo 135-8548, Japan (2F the Centennial Main Building, Toyosu Campus)
TEL:+81-(0)3-5859-7070 / FAX:+81-(0)35859-7071
E-mail:koho@ow.shibaura-it.ac.jp