Redox-Switchable Dyes Offer Tunable Visible to Near/Short-Wave-Infrared Fluorescence
- Research
Pyrazinacene-based compounds, designed with intramolecular charge transfer, offer tunable fluorescence for advanced bioimaging and optical applications
Near-infrared fluorescent dyes hold promise for applications like bioimaging, solar energy, and more, but their synthesis is often complex. Researchers at Shibaura Institute of Technology have developed redox-switchable pyrazinacene-based dyes using an intramolecular charge transfer design. This approach enables tunable fluorescence from the visible to near/short-wave-infrared regions and supports applications such as real-time redox sensing in cells. This is useful for monitoring cellular redox processes and imbalances.
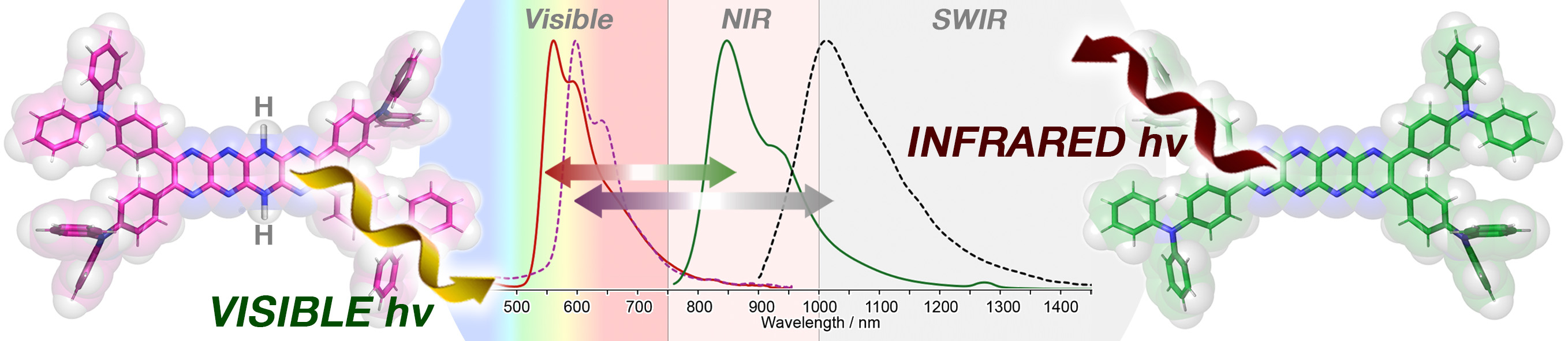
Title: Pyrazinacene dyes: A new class of redox-responsive fluorescent probes tunable for visible and infrared applications
Caption: Redox-active triphenylamine-appended pyrazinacenes show extreme reversible redox-coupled switching of their photophysical properties. In their reduced state, absorption and emission bands fall well within the visible region. Oxidation results in a shift in the absorption and fluorescence bands to the near-infrared and short-wave infrared regions while maintaining high fluorescence quantum yields.
Credit : Gary J. Richards and Akiko Hori from Shibaura Institute of Technology, Japan
Source Link: https://doi.org/10.1002/anie.202504564
License: CC BY 4.0
Usage restrictions: Credit must be given to the creator.
Fluorescent molecules that absorb and emit light in the near-infrared (NIR) and short-wave infrared (SWIR) regions have significant potential for various applications. These molecules can serve as markers for imaging biological tissues deep within the body, enhance solar cell efficiency by capturing more sunlight, or be incorporated into laser-protection eyewear to block harmful radiation from reaching the eyes. However, designing organic compounds with strong NIR emission is challenging. The energy absorbed by these molecules is often lost as heat due to vibrations in C-H bonds. To mitigate such losses and enhance fluorescence, heavier atoms like deuterium or fluorine need to be introduced into the molecular structure, which adds complexity to the synthesis process.
Researchers from Shibaura Institute of Technology have developed fluorescent dyes based on nitrogen-rich pyrazinacenes that exhibit strong, tunable fluorescence between the visible and NIR/SWIR regions. The study, published online in the journal Angewandte Chemie International Edition on March 03, 2025, was conducted by a multidisciplinary team led by Gary J. Richards of Shibaura Institute of Technology, as well as Kazushi Nakada, Toshiki Tajima, and Akiko Hori of the same institute, and Jonathan P. Hill of the National Institute for Materials Science.
“Infrared fluorescent molecules have great potential in areas like bioimaging and sensing, but strong emission at longer wavelengths is difficult to achieve with organic compounds,” explains Dr. Richards. “Our design enables compact molecular structures to exhibit dramatic red-shifted and switchable fluorescence through a simple redox process, which can be achieved either chemically or electrochemically.”
Pyrazinacenes with four or five rings can reversibly switch between two redox states: a fully oxidized form made entirely of pyrazine rings, and a reduced form that includes one dihydropyrazine ring. However, both states emit only within the visible range, limiting their usefulness for infrared applications. Larger pyrazinacenes, with six or seven fused rings, can emit light closer to the NIR range. However, these molecules tend to remain in a stable reduced state and do not oxidize easily, making reversible redox switching difficult.
To address these limitations, the researchers used a design strategy known as intramolecular charge transfer. This approach links an electron-donating group to an electron-accepting group through a π-conjugated molecular bridge, changing the charge distribution within the molecule. This internal charge transfer lowers the energy required for light absorption and emission, shifting the fluorescence toward longer wavelengths.
Using this strategy, the team added electron-donating triphenylamine groups to smaller pyrazinacenes, creating two new redox-active compounds: octaazatetracene (compound 1) and decaazapentacene (compound 2). In their reduced forms, compound 1 and compound 2 exhibited bright yellow and red fluorescence, respectively, with strong visible emissions at 560 nm and 599 nm, and quantum yields of 58% and 43%. When oxidized, their emission spectra shift to the NIR and SWIR regions, with emission wavelengths at 847 nm and 1,012 nm, and quantum yields of 16.4% and 1.4%, respectively. Notably, these values compare well with those of existing NIR and SWIR fluorescent dyes, highlighting their potential for imaging applications.
Such redox-responsive fluorescent molecules could be particularly useful for detecting changes in redox conditions within cells, which is crucial for diagnosing diseases like cancer, where tumors often exhibit distinct redox environments compared to healthy tissue. By combining nitrogen-rich pyrazinacenes with innovative molecular design, the researchers have established a versatile platform for creating fluorescent molecules that respond to redox changes.
“These compounds are the first examples of this strategy,” says Dr. Richards. “With further modifications of the donor groups, we expect to develop more efficient infrared dyes for a wide variety of applications.”
Reference
|
Title of original paper: |
Redox-Activated Near Infrared/Shortwave Infrared Emissive Chromophores: Synthesis of Triphenylamine-Appended Pyrazinacenes |
|
Journal: |
Angewandte Chemie International Edition |
|
DOI: |
Additional infotmation for EurekAlert
| Latest Article Publication Date: | 03 March 2025 |
| Method of Research: | Experimental study |
| Subject of Research: | Not applicable |
| Conflicts of Interest Statement: | The authors declare no conflict of interest. |
Authors
About Associate Professor Gary J. Richards from SIT, Japan
Dr. Gary J. Richards was an Associate Professor in the Department of Applied Chemistry at Shibaura Institute of Technology. His research specializes in structural and physical organic chemistry and organic functional materials, with a focus on pyrazinacenes, π-conjugated systems, supramolecular chemistry, and organic electronics. Dr. Richards holds a Ph.D. in Chemistry from the University of Hull and has held research positions at NIMS and Ochanomizu University.
About Professor Akiko Hori from SIT, Japan
Dr. Akiko Hori is a Professor at the Department of Applied Chemistry, Shibaura Institute of Technology (SIT). She heads the Laboratory of Molecular Assemblies. Her research is focused on crystal engineering and supramolecular chemistry based on the combination of inorganic and organic materials.
Funding Information
This work was supported by Grants-in-Aid for Scientific Research C, no. 21K05044 and 24K08401 (G.J.R.), and parts of this work were supported by Grant-in-Aids for Scientific Research B, no. 21H01955 and 23K21122 (A.H.) of JSPS KAKENHI and by World Premier International Research Center Initiative (WPI Initiative), MEXT, Japan (J.P.H.).