Treating Prostate Cancer with Novel Platinum Complex via Targeting Androgen Receptor Signaling
- Research
A new azolato-bridged platinum complex 5-H-Y demonstrates strong antiproliferative effects with low toxicity in preclinical models
Prostate cancer, especially in advanced stages such as castration-resistant prostate cancer, is challenging to treat. Traditional therapies targeting androgen receptor (AR) signaling have limited efficacy. In a recent study, Japanese researchers investigated the potential of azolato-bridged dinuclear platinum(II) complexes, particularly 5-H-Y, as promising alternatives. Their findings highlight the complex’s ability to inhibit AR signaling and induce cell death in prostate cancer cells, providing a new avenue for prostate cancer treatment.
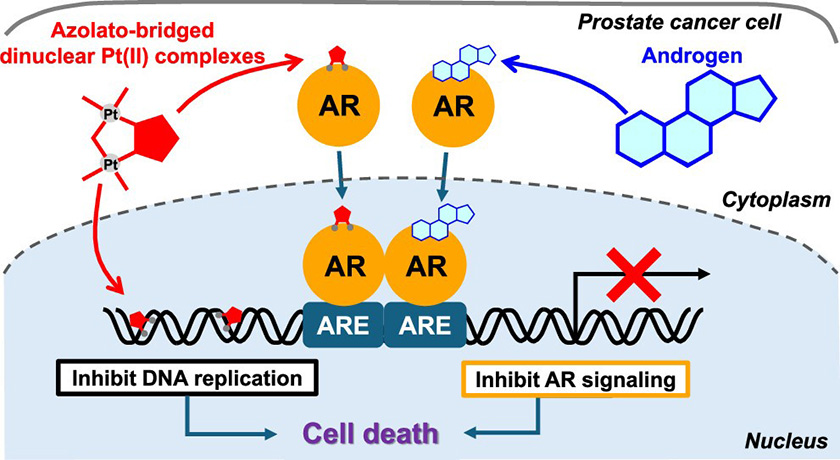
Title: Mechanism of action of dinuclear platinum(II) complexes.
Caption: The dinuclear platinum(II) complexes inhibit prostate cancer cell growth by binding to the androgen receptor (AR) and disrupting AR-mediated gene expression. This mechanism leads to apoptosis and prevents dihydrotestosterone (DHT)-induced cell proliferation.
Credit: Yoshihisa Hirota from SIT, Japan
Source: https://doi.org/10.1021/acs.inorgchem.4c01093
License type: Original Content
Usage restrictions: Cannot be used without permission.

Title: Multi-layered attack on prostrate cancer with a single Pt(II) complex.
Caption: Dinuclear platinum (II) complexes, particularly 5-H-Y, show promising anticancer activity against prostate cancer by inhibiting androgen receptor signaling and inducing apoptosis, offering a potential alternative to conventional treatments such as cisplatin.
Credit: Inorganic Chemistry
License type: Original Content
Usage restrictions: Cannot be used without permission.
Prostate cancer remains a global health challenge, ranking as the second most commonly diagnosed cancer among men. Although treatments like androgen deprivation therapy have been effective for early-stage prostate cancer, advanced stages, such as castration-resistant prostate cancer, present significant treatment challenges due to resistance to therapies. Current approaches targeting androgen receptor (AR) signaling, such as taxanes and newer agents, show limited success. Cisplatin, a widely used anticancer drug, has been used in combination therapies but its use is limited by severe side effects, including renal toxicity, highlighting the need for safer and more effective treatment options.
In a recent study published in Volume 63, Issue 44 of the journal Inorganic Chemistry on September 11, 2024, a team of researchers led by Associate Professor Yoshihisa Hirota from Shibaura Institute of Technology (SIT) and Professor Seiji Komeda from Suzuka University of Medical Science, explored the potential of azolato-bridged dinuclear platinum(II) complexes (azolato-bridged complexes) in treating prostate cancer. The study particularly focused on a complex called 5-H-Y ([{cis-Pt(NH3)2}2(μ-OH)(μ-tetrazolato-N2,N3)](ClO4)2) as an alternative to cisplatin. These complexes are characterized by their water solubility and promising antiproliferative effects against prostate cancer cell lines, with minimal toxicity compared to traditional platinum-based drugs.
“The first platinum-based drug, cisplatin, has a powerful effect on cancer by binding to nuclear DNA, but it also affects normal cells and can cause serious side effects. We had data showing that some azolato-bridged complexes inhibit AR signaling, which is extremely important for prostate cancer proliferation, in addition to the anticancer effect initiated by the DNA-binding. Therefore, this study was conducted to clarify the mechanism of AR signaling inhibition by the azolato-bridged complex, 5-H-Y..,” explains Dr. Hirota.
The team used a variety of methods to evaluate AR dynamics and therapeutic effects in LNCaP prostate cancer cells. They utilized azolato-bridged complexes, cisplatin, and the AR antagonist KW-365 to explore their efficacy and performed cell viability, gene expression, and protein analyses. Additionally, the team employed immunofluorescence staining to visualize AR expression and evaluated apoptosis (programmed cell death), cell cycle distribution, and nuclear platinum accumulation.
The results showed that 5-H-Y exhibited significantly stronger cytotoxic effects than cisplatin, with a low half-maximal inhibitory concentration for dihydrotestosterone (DHT)-induced cell proliferation. Moreover, 5-H-Y effectively suppressed the expression of AR-responsive genes, such as PSA and TMPRSS2, and induced apoptosis in AR-overexpressing cells. Immunofluorescence analysis confirmed that 5-H-Y promoted chromatin fragmentation, a hallmark of apoptosis, with greater efficacy observed at higher concentrations.
Mechanistically, 5-H-Y was found to bind directly to AR and DNA through both noncovalent and covalent interactions. This binding induced conformational changes in AR, potentially disrupting its function. Additionally, 5-H-Y arrested cell cycle in the G2/M and sub-G1 phases, leading to apoptosis, particularly in AR-overexpressing cells. This multimodal mechanism of action distinguished 5-H-Y from cisplatin, which primarily targets DNA.
Despite its high antiproliferative activity, however, 5-H-Y demonstrated lower acute toxicity in vivo compared to other platinum complexes, making it a promising candidate for further development. “The azolato-bridged complexes used in this research are expected to play a key role in developing new treatments for advanced prostate cancer. For patients whose cancer has become resistant to conventional therapies, these complexes have the potential to effectively inhibit cancer progression with multi-layered attack while minimizing side effects. Our approach could thus expand treatment options for prostate cancer and improve the patient’s quality of life,” concludes Dr. Hirota enthusiastically.
Overall, the results suggest that dinuclear platinum(II) complexes could offer a more targeted approach to treating prostate cancer, specifically by inhibiting AR-mediated cell growth and survival, paving the way for the development of new, more effective therapies for advanced prostate cancer.
Reference
| Title of original paper: |
Azolato-Bridged Dinuclear Platinum(II) Complexes Exhibit Androgen Receptor-Mediated Anti-Prostate Cancer Activity |
| Journal: | |
| Article link: |
Authors
About Associate Professor Yoshihisa Hirota from SIT, Japan
Yoshihisa Hirota is an Associate Professor at the Shibaura Institute of Technology, affiliated with the Department of Bioscience and Engineering, College of Systems Engineering and Science. He holds a Ph.D. in Pharmaceutical Sciences from Kobe Pharmaceutical University and has conducted international research as a postdoctoral fellow in the U.S. (University of Cincinnati, School of Medicine). Dr. Hirota’s research focuses on Medicinal Science and Nutritional Biochemistry, with particular emphasis on the roles of fat-soluble vitamins and nucleic acids in biological systems. With 42 publications to his name, Dr. Hirota’s expertise bridges molecular biology, nutrition, with the goal of extending the healthy life expectancy of the world, he will continue to promote research into nutrients and medicine, and lead to better healthcare solutions.
About Professor Seiji Komeda from Suzuka University of Medical Science, Japan
Funding Information
This work was partly supported by the Japan Society for the Promotion of Science (KAKENHI Grant Numbers 19K07018, 23K06074, 18K11056, 21K11709, 24K14656, and Grant for the Promotion of Joint International Research 18KK0455).