Successful Development of Pauson–Khand Reaction with Atropisomeric Substrates
- Research
The reaction can serve as a novel method for transforming atropisomers into specific enantiomers with desirable properties
Atropisomers are a class of isomers that form as a result of restricted rotation around a single bond in a molecule. These compounds are considered valuable for the synthesis of pharmaceutically relevant enantiomers. Now, for the first time, researchers have utilized Pauson–Khand reaction to convert enantioenriched atropisomeric sulfonamides into chiral nitrogen-containing tricyclic compounds. Findings of this study are expected to advance the synthesis of natural products and bioactive compounds.
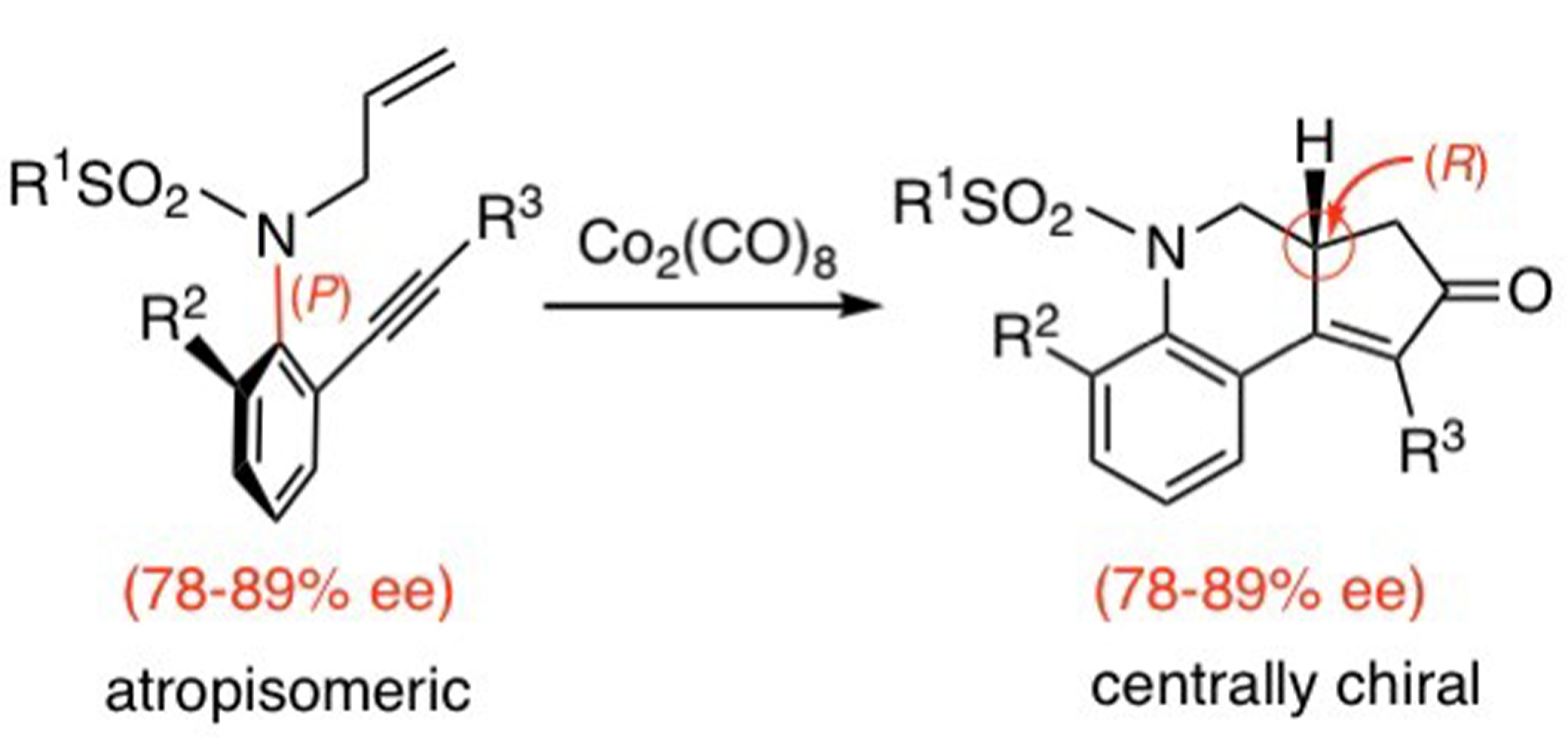
Image Title: Pauson−Khand reaction with enantioenriched N−C axially chiral sulfonamide derivatives
Image Caption: In a new study, researchers demonstrate that Pauson–Khand reaction can be applied to atropisomeric substrates for transferring chirality from the chiral axis to a center. The reaction can be applied for the synthesis of natural products and bioactive compounds in the future.
Image Credit: Osamu Kitagawa from SIT, Japan
License Type: Original Content
Usage Restrictions: Cannot be reproduced without permission
The rich diversity of organic compounds is a result of the remarkable ability of carbon atoms to connect and form bonds with different molecules. Variations in these bonding arrangements, as well as the types of atoms and functional groups involved, result in the formation of isomers. They are a type of compounds that share the same molecular formula but exhibit distinct three-dimensional shapes and properties.
Atropisomers are a type of isomers that are formed when bulky substituents or functional groups get attached to a single bond and experience restricted rotation about the bond. This restriction leads to molecules with a distinct spatial arrangement of atoms and functional groups. Atropisomers are often encountered in compounds containing aromatic rings. Notably, compounds formed as a result of restricted rotation around an N−C single bond between the aromatic ring and the functional group are used as chiral ligands and chiral building blocks to create specific stereoisomers with chiral carbon centers. They often find use in various chemical and pharmaceutical applications. However, maintaining axial chirality around the N−C bond is challenging to attain, and this results in a limited number of synthesizable N−C axially chiral compounds.
In a recent study, researchers have now demonstrated that the Pauson–Khand reaction—a chemical reaction for synthesizing molecules containing cyclopentenones—when applied to atropisomeric substrates, can produce new derivatives containing a chiral carbon. The study was made available online on 2 October 2023 and published in the journal Organic Letters on 13 October 2023. It was led by Professor Osamu Kitagawa from the Department of Applied Chemistry at Shibaura Institute of Technology, Japan along with graduate students Ryohei Kasahara and Tatsuya Toyoda. Possibly the first demonstration of this kind, this breakthrough significantly expands the scope of synthesizing such compounds.
“Although Pauson–Khand reaction is a widely used reaction for the synthesis of many organic compounds, the reaction with atropisomeric substrates had not been explored yet. Out of academic curiosity, we decided to explore the Pauson–Khand reaction with atropisomeric substrates,” says Prof. Kitagawa, while talking about the study.
To this end, the researchers applied an intramolecular version of the reaction to enantioenriched atropisomeric sulfonamides for synthesizing chiral nitrogen-containing tricyclic compounds. They observed that conducting Pauson–Khand reactions on enantioenriched atropisomeric sulfonamides (78–89% ee) containing various substituents in the presence of a Co2(CO)8 complex led to the formation of products with enantiomeric excesses ranging from 78% to 89%. These findings suggest that the Pauson–Khand reaction can effectively transfer chirality from the starting materials to the reaction products with a high degree of selectivity.
“The reaction with enantioenriched N–C axially chiral sulfonamide derivatives proceeded with complete chirality transfer from axial chirality (P configuration) to central chirality (R configuration), affording chiral nitrogen-containing tricyclic compounds,” explains Prof. Kitagawa.
Further, a wide range of substrates were compatible with the reaction, including sulfonamides with methyl-, chloro-, and bromo- substituents, as well as aromatic groups. A crucial factor for the success of this approach was the presence of the Co2(CO)8 complex, which forms an intermediate with the substrate. The specific arrangement of carbon and cobalt atoms within this intermediate controls how alkenes attach to it, promoting the formation of specific enantiomers.
In summary, this level of control over chirality is of significant importance in chemical synthesis and has practical applications in various fields, particularly for the synthesis of pharmaceutical compounds. “With the reaction products possessing a nitrogen-containing tricyclic structure, the reaction can be used for novel applications, such as for the synthesis of natural products and bioactive compounds,” concludes Prof. Kitagawa.
Reference
| Title of original paper: |
Chirality Transfer Intramolecular Pauson−Khand Reaction with N−C Axially Chiral Sulfonamides Bearing an Ene−Yne Structure |
| Journal | |
| Article link: | 10.1021/acs.orglett.3c02893 |
Funding Information
This work was partly supported by JSPS KAKENHI (C20K06945).